Diagnostic Tests for Ehrlichia and Anaplasma
A clinical diagnosis of Ehrlichia (HME) or Anaplasma (HGA) infection may be difficult because symptoms can vary significantly from patient to patient. However, an Ehrlichia test or Anaplasma test from IGeneX can help physicians accurately diagnose HME or HGA. If you’re experiencing symptoms of a tick-borne illness, review the guide below to determine which IGeneX test to order.
WHAT TESTS ARE AVAILABLE FOR EHRLICHIA AND ANAPLASMA?
IGeneX offers IFAs (Indirect Immunofluorescent Assay) and PCRs (Polymerase Chain Reaction), and Culture Enhanced PCR (cePCR) to confirm the diagnosis of Ehrlichia and Anaplasma.
IgG and IgM Antibody for Ehrlichia (HME and HGA) by IFA
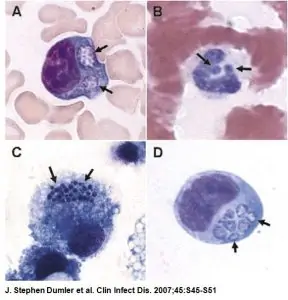
The Ehrlichia Immunofluorescence Assays (IFAs), Anaplasma phagocytophilum (HGA) IFA and Ehrlichia chaffeensis (HME) IFA, are designed to test human serum for IgG and IgM antibodies to Ehrlichia HGA and HME antigens, respectively. For diagnostic purposes, HGA and HME IFA test results should be used in conjunction with other information available to the diagnosing physician.
Principle
The Ehrlichia IFA assay is a two-stage sandwich assay, based upon an antigen-antibody complex formation in the following steps:
- Binding of anti-Ehrlichia specific antibodies in human serum to fixed
- Binding of fluorescent-labeled anti-human IgG/IgM antibodies to the human anti-Ehrlichia antibodies bound to Ehrlichia on the slide.
- Identifying green fluorescing Ehrlichia organisms with a fluorescent microscope.
Note: For HGA IFA, A. phagocytophilum is fixed to the slide. For HME IFA, Ehrlichia chaffeensis is fixed to the slide.
Reference Range
- HME or HGA antibody titers IgM <20
- HME or HGA antibody titers IgG <40
Clinical Significance
The Ehrlichia chaffeensis (HME) IFA is an Ehrlichia test used to detect antibodies to Ehrlichia chaffeensis in human serum, while the Anaplasma phagocytophilum (HGA) IFA is an Anaplasma test used to detect antibodies to Anaplasma phagocytophilum in human serum. Titers rise during the first two-to-four weeks of illness and decline over the next six-to-12 months.
In patients with previously high titers, the presence of an IgG titer of fewer than 160 may indicate a resolving infection. If the IFA result is negative but the clinical symptoms of HME or HGA infection are present, PCR testing is suggested.
Finally, E. chaffeensis and A. phagocytophilum are carried by the same ticks that have also been known to cause Babesiosis, Bartonellosis and Lyme disease. Patients with positive titers should also be tested for other tick-borne diseases.
Limitations
- A negative HME IFA test result does not exclude the possibility of chaffeensis
- A negative HGA IFA test result does not exclude the possibility of phagocytophilum
- Cross-reactions can occur among the Rickettsiaceae, including: Rickettsia, Ehrlichia and Anaplasma.
- Ehrlichia and Anaplasma test results should be interpreted in conjunction with other laboratory and clinical findings.
Stage of Disease
Any Stage of Disease (Early to Late/Chronic Stage)
Results Interpretations >
View Ehrlichia and Anaplasma IFA tests >
Ehrlichia PCR
The polymerase chain reaction (PCR)-based diagnostic assays for detection of Ehrlichia from blood are highly specific to Anaplasma phagocytophilum (HGA) and Ehrlichia chaffeensis (HME) DNAs. The combination of the following three steps imparts a very high specificity and sensitivity to these Ehrlichia and Anaplasma tests:
- Hybridization/selection
- Amplification of Ehrlichia-specific DNA
- Detection of Ehrlichia-specific amplified DNA fragments
Reference Range
- Ehrlichia chaffeensis (HME):
Negative, chaffeensis-specific DNA not detected - Anaplasma phagocytophilum (HGA): Negative, phagocytophilum-specific DNA not detected
Clinical Significance
The Ehrlichia chaffeensis (HME) PCR test detects E. chaffeensis ribosomal DNA (rDNA) fragments in patient samples. The A. phagocytophilum (HGA) PCR test detects A. phagocytophilum rDNA fragments in patient samples. The Ehrlichia or Anaplasma rDNA fragments are hybrid-selected by probes, followed by PCR amplification of the selected fragments using specific primers. PCR products are then confirmed with E. chaffeensis– and A. phagocytophilum-specific probes by southern blot assay.
Limitations
- Ehrlichia and Anaplasma test results should be interpreted in conjunction with other laboratory and clinical findings.
- Test results can only help the physician in confirming a clinical diagnosis.
Stage of Disease
Any Stage of Disease (Early to Late/Chronic Stage)
Results Interpretations >
View Ehrlichia and Anaplasma PCR tests >
Ehrlichia and Anaplasma cePCR - New!
Culture testing is widely considered to be the “gold standard” for diagnosis of Ehrlichia and Anaplasma. For many years, Ehrlichia and Anaplasma cultures were too expensive and tedious to be practical for laboratory use. Until now. After many years of research and development, IGeneX is pleased to introduce cePCR (Culture-Enhanced PCR) for Ehrlichia and Anaplasma.
Download the cePCR datasheet >
Principle
In culturing, a clinical sample from the body (e.g. blood) is incubated in media.
During this incubation period, micro-organisms in the sample grow and multiply. The sample is then tested by PCR to identify the pathogens.
Advantages of Culture-Enhanced PCR
• Provides higher sensitivity than standard PCR testing.
• The only 100% specific method for identification of Ehrlichia and Anaplasma.
• Obtaining cultures before antibiotic use improves the chances of identifying the offending microorganism, which improves patient care.
Stage of Disease
Any stage
Complete Test Directory
THE DIRECTORY
GET STARTED TODAY!
The first step in getting tested with IGeneX is to order a collection kit. Choose between a Blood, Urine, or Miscellaneous kit. Doctors can order unlimited quantities of kits at no charge. Patients are required to deposit $20, which is applied to the testing fees.